
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.47SP
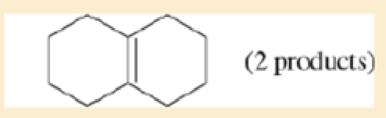
For each compound, predict the major product of free-radical bromination. Remember that bromination is highly selective, and only the most stable radical will be formed.
- a. cyclohexane
- b. methylcyclopentane
- c. decalin
- d. hexane
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A reaction flask contains 2-bromopentane in an ethanolic solution of sodium ethoxide at room temperature and result in the formation of two olefnic products. What is responsible for the formation of major and minor products. A. Different activated complex involved in the mechanism. B. Bimolecular nucleophilic substitution reaction. C. Bimolecular elimination reaction D. The presence of sodium ethoxide. E. The hybridization nature of secondary carbocation
Explosions occur when the rate of reaction increases dramatically over a short period of time. There are several types of explosions such as autocatalytic explosions, thermal explosions and branched chain explosions.
a. Briefly discuss how the thermal explosion and branched chain explosion occur.
6. Select the incorrect statement conceming the reaction you conducted during the
isomerization experiment. Choose one.
H,CO.
Brz, light
H,CO.
rOCH
rOCH
methylene chioride
dimethyl maleate
dimethyl fumarate
A. The major intermediate formed during the reaction contains both a bromine atom
and a radical.
B. Bromine is added catalytically instead of stoichiometrically because the bromine
radical is regenerated when the product is formed.
C. After twenty minutes, the reaction mixture contained both reactant and product.
D. Both reactant and product are soluble in methylene chloride.
E. If the reaction mixture turned colorless, that was an indication that the bromine
radicals were consumed.
F. When cold hexanes was added, only dimethyl fumarate precipitated out of
solution because dimethyl maleate is soluble in cold hexanes.
Chapter 4 Solutions
Organic Chemistry (9th Edition)
Ch. 4.3A - Draw Lewis structures for the following free...Ch. 4.3B - a. Write the propagation steps leading to the...Ch. 4.3C - Prob. 4.3PCh. 4.3C - Prob. 4.4PCh. 4.4 - The following reaction has a value of G =...Ch. 4.4 - Under base-catalyzed conditions two molecules of...Ch. 4.5B - When ethene is mixed with hydrogen in the presence...Ch. 4.5B - For each reaction, estimate whether S for the...Ch. 4.7 - a. Propose a mechanism for the free radical...Ch. 4.7 - a. Using bond-dissociation enthalpies from...
Ch. 4.8 - The reaction of tert-butyl chloride with methanol...Ch. 4.8 - Under certain conditions, the bromination of...Ch. 4.8 - When a small piece of plat num is added to a...Ch. 4.10 - Prob. 4.14PCh. 4.10 - Prob. 4.15PCh. 4.12 - The bromination of methane proceeds through the...Ch. 4.12 - a. Using me BDEs in Table4-2 (page 167 ), compute...Ch. 4.13A - What would be the product ratio in the...Ch. 4.13A - Classify each hydrogen atom in the following...Ch. 4.13B - Use the bond-dissociation enthalpies in Tabte4-2...Ch. 4.13B - Prob. 4.21PCh. 4.13B - Prob. 4.22PCh. 4.14 - a. Compute the heats of reaction for abstraction...Ch. 4.14 - 2,3-Dimethylbutane reacts with bromine in the...Ch. 4.14 - Prob. 4.25PCh. 4.15 - Prob. 4.26PCh. 4.15 - Prob. 4.27PCh. 4.16A - Prob. 4.28PCh. 4.16A - Prob. 4.29PCh. 4.16B - Prob. 4.30PCh. 4.16C - Prob. 4.31PCh. 4.16C - Acetonitrile (CH3C N) is deprotonated by very...Ch. 4.16D - Prob. 4.33PCh. 4 - The following reaction is a common synthesis used...Ch. 4 - Consider the following reaction-energy diagram. a....Ch. 4 - Draw a reaction-energy diagram for a one-step...Ch. 4 - Draw a reaction-energy diagram for a two-step...Ch. 4 - Prob. 4.38SPCh. 4 - Treatment of tert-butyl alcohol with concentrated...Ch. 4 - Label each hydrogen atom in the following...Ch. 4 - Prob. 4.41SPCh. 4 - Prob. 4.42SPCh. 4 - Prob. 4.43SPCh. 4 - Prob. 4.44SPCh. 4 - Prob. 4.45SPCh. 4 - Prob. 4.46SPCh. 4 - For each compound, predict the major product of...Ch. 4 - When exactly 1 mole of methane is mixed with...Ch. 4 - Prob. 4.49SPCh. 4 - Prob. 4.50SPCh. 4 - Prob. 4.51SPCh. 4 - When dichloromethane is treated with strong NaOH,...Ch. 4 - Prob. 4.53SPCh. 4 - When a small amount of iodine is added to a...Ch. 4 - Prob. 4.55SPCh. 4 - When healthy, Earths stratosphere contains a low...Ch. 4 - Prob. 4.57SPCh. 4 - lodination of alkanes using iodine (I2) is usually...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What kind of reaction is an 'Grignard reaction'? Also What is the critical intermediate that can be isolated?arrow_forwardUse the dropdown menu to indicate whether the rate of the reaction shown below will increase, decrease, or remain the same when the reaction conditions are changed to X or to Y or to Z (see below). NaCN Br CN CH3CN X: Change the leaving group from Br to CI Y: Increase the concentration of haloalkane Z: Increase the concentration of NaCN X: choose your answer... Y: choose your answer... Z: choose your answer... >arrow_forwardThe oxymercuration or the demercuration of this compound will form what product? A. 1-methylcyclohexan-1-ol/-methyl-1-cyclohexanol B. 1-methylcyclohexan-2-ol/-methyl-2-cyclohexanol C. 1-methylcyclohexan-3-ol/-methyl-3-cyclohexanolarrow_forward
- 1. Free radicals are generated by subjecting a molecule such as chlorine or bromine gas to A. high temperature conditions. B. photolysis. C. ionizing conditions. D. choices A and B 2. Which of the following radical intermediates is the most stable? q •CF3 A. B. C. D. 3. In the reaction shown below the intermediate that is formed at the fastest rate is Cl₂ A. uv CH, CH₂CHCH₂CH₂CH₂ B. CH, CH.CCH₂CH₂CH, CH₂ C. CH.CHCHCH.CH, D. CH₂ CH₂CHCH₂CHCHarrow_forwardAs we will learn in Section 15.12, many antioxidants-compounds that prevent unwanted radical oxidation reactions from occurring-are phenols, compounds that contain an OH group bonded directly to a benzene ring. a. Explain why homolysis of the O-H bond in phenol requires considerably less energy than homolysis of the O-H bond in ethanol (362 kJ/mol vs. 438 kJ/mol). b. Why is the C-O bond in phenol shorter than the C-O bond in ethanol? -O-H CH,CH2-0-H phenol ethanolarrow_forwardAs we will learn in Section 13.12, many antioxidants–compounds that prevent unwanted radical oxidation reactions from occurring–are phenols, compounds that contain an OH group bonded directly to a benzene ring. a. Explain why homolysis of the O–H bond in phenol requires considerably less energy than homolysis of the O–H bond in ethanol (362 kJ/mol vs. 438 kJ/mol). b.Why is the C–O bond in phenol shorter than the C–O bond in ethanol?arrow_forward
- Match the species with the description of its reactivity. alkene chloride 1. nucleophile carbocation 2. electrophile HCIarrow_forwardDefine " reaction mechanism." Define "nucleophile." What electronic features characterize a nucleophile? Define "electrophile." What electronic features characterize an electrophile?arrow_forwardYou determine the bimolecular rate constant k for a set of SN2 reactions. Based on your understanding of how the nucleophile effects the rate constant for an SN2 reaction, drag and drop the nucleophiles into the correct rows in the table below. Alkyl halide Nucleophile k / L mol-1 s-1 CI 0.011 -CI 0.045 0.074 0.095 -NH2 -NH2 >-NH2 -NH2 -NH2arrow_forward
- a.What carbon radical is formed by homolysis of the C–Ha bond in propylbenzene? Draw all reasonable resonance structures for this radical. b.What carbon radical is formed by homolysis of the C–Hb bond in propylbenzene? Draw all reasonable resonance structures for this radical. c. The bond dissociation energy of one of the C–H bonds is considerably less than the bond dissociation energy of the other. Which C–H bond is weaker? Offer an explanation.arrow_forwardWrite a radical mechanism monobromination for the following compounds: a. Cyclopentane to form bromocyclopentane b. butane to form 2-bromobutane c. methylcyclopentane to form 1-bromo-1-methylcyclopentanearrow_forwardWhich is/are NOT TRUE about bimolecular nucleophilic substitution reactions? Select one or more: 1. A carbocation intermediate is formed. 2. A strong nucleophile displaces a halogen atom in a concerted mechanism. 3. Presence of polar aprotic solvents promotes this reaction. 4. Methyl halides react faster than secondary alkyl halides.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License