
Concept explainers
Interpretation:
The leaving group in the given molecule is to be determined inorder identify the positively charged and negatively charged fragments produced during mechanism.
Concept Introduction:
Heterolytic cleavage of sigma bond occurs under a variety of conditions. The mechanism of bond cleavage is as shown below,
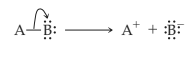
The arrow in the above mechanism indicates that the sigma electrons form A-B bond are leaving A and becoming the exclusive property of B. Since the fragment A is formally losing one electron, it must become positively charged and B must become negatively charged since it gains an electron.
Answer to Problem 1EQ
The mechanism yields a negatively charged Chloride ion and positively charged isopropyl cation.
Explanation of Solution
The given Lewis structure of isopropyl chloride:
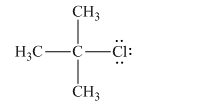
Chlorine is the leaving group in this case. As Chlorine is more electronegative, it obtains the shared electron pair during heterolytic bond cleavage as shown below.
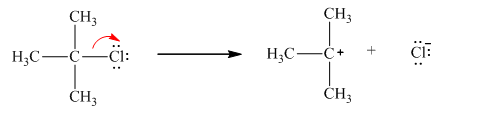
Hence, the cleavage of isopropyl chloride yields chloride ion (negatively charged as shared electron is obtained) and isopropyl cation.
The positive and negative fragments produced in the mechanism are identified.
Want to see more full solutions like this?
Chapter 3 Solutions
Pushing Electrons
- Define " reaction mechanism." Define "nucleophile." What electronic features characterize a nucleophile? Define "electrophile." What electronic features characterize an electrophile?arrow_forwardQuestion : Activation energies will be greater than the heat of reaction in endothermic reactions that involve: A. Both bond formation and bond rupture B. Both bond formation and bond breaking C. Bond formation D. bond breaking E. None of the abovearrow_forward1. Which among these can make a molecule nucleophilic? a.double bondsb.positive chargec. incomplete octet 2. Which among these can make a molecule electrophilic? a.Triple bondsb.positive chargec. radicalsarrow_forward
- 3. Why did breaking the P-C single bond lead to the formation of 2 species containing a free radical (ie. where did those 2 electrons come from)? 4. Do you think the photoinitiator 2-hydroxy-2-methylpropiophenone would require a shorter or longer wavelength of light to create a free radical? Explain your answer using the table provided in question #2 (be specific). The structure of 2-hydroxy-2- methylpropiophenone is shown below. CH3 Figure 5: Structure of 2-hydroxy-2- methylpropiophenone. The wavy line shows OH the bond that is photo-sensitive (ie. the bond that will be broken by light). (Taken from Tehfe et al. 2013, http://www.mdpi.com/2076- 3417/3/2/490/htm) ČH3 5. Why is the PEGDA monomer soluble in water, but the polymer is not? In other words, why do you think forming a large molecule would lead to the formation of a solid? (Hint: think about the movement of molecules in a liquid vs solid phase). 6. Thinking about the octet rule, why do you think many free radicals are very…arrow_forward1. _________ FeBr3 + ______H2SO4 -----> _____Fe2(SO4)3 + ____HBr Show work on how you solve this.arrow_forward5. Consider the reaction below and answer the following questions + OH + H₂O a. Draw in ALL missing lone pair electrons as well as electron movement arrows on the reactant side Identify the nucleophile and electrophile on the reactant side b. c. Identify which side of the equilibrium will dominate. d. Draw resonance structures for the organic compound on the product side CHEM 241-Group05. Ormord d ****arrow_forward
- In the substitution reaction: The product(s) of the reaction is(are)? Select all that are correct.arrow_forwardZ ZI OCH 3arrow_forward"Nucleophilic substitution reaction"" When does the bond between the leaving group and C break? Does it break at the same time that the new bond between the nucleophile and C forms? Or Does the bond to the leaving group break first.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
