
Concept explainers
Interpretation:
The leaving group in the given molecule is to be determined in order identify the positively charged and neutral fragments produced during mechanism.
Concept Introduction:
Heterolytic cleavage of sigma bond occurs under a variety of conditions. The mechanism of bond cleavage is as shown below,
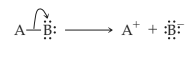
The arrow in the above mechanism indicates that the sigma electrons form A-B bond are leaving A and becoming the exclusive property of B. Since the fragment A is formally losing one electron, it must become positively charged and B must become negatively charged since it gains an electron.
Often, heterolytic cleavage occurs from a charged intermediate that was formed in a previous step. The process is the same, but the charge on the products is different.

Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Pushing Electrons
- Why does the dehydration of an alcohol more often use concentrated sulfuric acid, H2SO4H2SO4, as the acid catalyst rather than dilute hydrochloric acid, HCl? Select one or more: Hydrochloric acid is too small to effectively react with the alcohol in the reaction. The additional water solvent from a dilute solution could reverse the dehydration reaction. Only acids with more than one proton can complete the dehydration reaction. The presence of the chloride ion could result in a competing substitution reaction.arrow_forwardPAP Chemistry-2903012-42100P-1/ Le Chatelier's Principle/ Lesson 128 2. Zinc (Zn) granules react slowly with dilute hydrochloric acid (HCI), but much faster if the acid is concentrated. Zn(s) 2HCI(aq)ZnCl2(aq) + H2(g) Zinc + Hydrochloric Acid Zinc Chloride + Hydrogen What causes the reaction to proceed faster with concentrated acid? The concentrated hydrochloric acid causes more hydrogen gas to be produced. The pressure of hydrogen gas molecules increases as concentration increases. The concentrated hydrochloric acid molecules move faster than in dilute acid. There are more collisions between the zinc and concentrated hydrochloric acid. PREVIOUarrow_forwardDraw the condensed structure of the organic molecule that produces sodium propanoate when reacted with NaOH. Correct each molecule in the drawing area below so that it has the structure it would have if it were dissolved in a 0.1 M aqueous solution of NaOH. If there are no changes to be made, check the No changes box under the drawing area. No changes. HO–CH,—CH, NH HO—CH, OH C™ X с Oarrow_forward
- Consider this reaction: Br CH3OH Br-Br H3CO The mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the final product. In cases that involve a negatively charged nucleophile, the attack of the nucleophile leads directly to the product. H. Br + CH;OH Br Intermediate 2 (product) Intermediate 1 In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction. OH + Br2 + HBr Br racemic mixturearrow_forwardComplete the reaction scheme below by drawing the missing structure. If there is no structure possible that would produce the outcome shown, indicate that fact in the answer file that you submit. N-H 1) NaOH, H₂O 2) Br NaOH, H₂O heat "NH₂ oNa Naarrow_forwardPotassium permanganate and potassium dichromate are very similar in their oxidizing abilities, however there are differences. If I want to convert 4-hexen-1-ol into 4-hexenoic acid, which would be the appropriate oxidizing agent to use? Explain your answer using equations that show the two different products that would form via the two different oxidizing agents.arrow_forward
- Draw structural formulas for organic products A and B in the window below. Cul H₂C=CHBr Li pentane A ences B • Draw only products having the organic portion of the original alkyl halide. • Draw carbon-lithium bonds using the single bond tool. If a structure has a copper-lithium bond, do not draw the lithium. • Separate products from different steps using the → sign from the drop-down menu.arrow_forwardIn an esterification reaction, a carboxylic acid reacts with an excess of alcohol in acidic conditions to form an ester. Draw the structure of the ester product in the reaction between pentanoic acid and 1‑propanol. pentanoic acid+ 1-propanol= ester+ H20 structure of ester product neededarrow_forward4. Write the products of the reaction of butyric acid with the hydroxide ion (a base) shown below. HO + OHarrow_forward
- What is the acid and conjugated acid in this reaction H2SO4 + NH3 → HSO4ˉ + NH4⁺?arrow_forwardPredict the Product. Predict the major organic product(s) or reactant(s) for the following reactions. HO Fr Iz NaOH, H₂O, heat NaOH, H₂O, heatarrow_forwardDraw structural formulas for organic products A and B in the window below. ● ● ● -CH₂CI Li pentane Cul ChemDoodleⓇ Draw only products having the organic portion of the original alkyl halide. Draw carbon-lithium bonds using the single bond tool. If a structure has a copper-lithium bond, do not draw the lithium. Separate products from different steps using the → sign from the drop-down menu. B Sn [Farrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
